Raman Spectroscopy
Introduction
Raman spectroscopy is the measurement of the wavelength and
intensity of inelastically scattered
light from molecules. The Raman scattered light occurs at wavelengths that are
shifted from the incident light by the energies of molecular vibrations. The
mechanism of Raman scattering is different from that of infrared
absorption, and Raman and IR spectra provide complementary information.
Typical applications are in structure determination, multicomponent qualitative
analysis, and quantitative analysis.
Theory
The Raman scattering transition moment is:
R = <
Xi | a | Xj >
where Xi and Xj
are the initial and final states, respectively, and a is the polarizability of
the molecule:
a = ao + (r-re)(da/dr) + ... higher
terms
where r is the distance between atoms and ao is the
polarizability at the equilibrium bond length, re. Polarizability can
be defined as the ease of which an electron cloud can be distorted by an
external electric field. Since ao is a constant and <
Xi | Xj > = 0, R simplifies to:
R = <
Xi | (r-re)(da/dr) | Xj >
The result is
that there must be a change in polarizability during the vibration for that
vibration to inelastically scatter radiation.
Examples of Raman active and inactive vibrations in
CO2
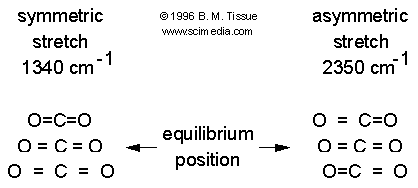
The polarizability depends on how tightly the electrons are bound to the
nuclei. In the symmetric stretch the strength of electron binding is different
between the minimum and maximum internuclear distances. Therefore the
polarizability changes during the vibration and this vibrational mode scatters
Raman light (the vibration is Raman active). In the asymmetric stretch the
electrons are more easily polarized in the bond that expands but are less easily
polarized in the bond that compresses. There is no overall change in
polarizability and the asymmetric stretch is Raman inactive.
Raman line intensities are proportional to:
nu4 * sigma(nu) * I
* exp(-Ei/kT) * C
where nu is the frequency of the incident
radiation, sigma(nu) is the Raman cross section (typically 10-29
cm2), I is the radiation intensity, exp(-Ei/kT) is the
Boltzmann factor for state i, and C is the analyte concentration.
Instrumentation
The most common light source in Raman spectroscopy is an
Ar-ion laser. Resonance Raman spectroscopy requires tunable radiation and
sources are Ar-ion-laser-pumped cw dye lasers, or high-repetition-rate
excimer-laser-pumped pulsed dye lasers. Because Raman scattering is a weak
process, a key requirement to obtain Raman spectra is that the spectrometer
provide a high rejection of scattered laser light. New methods such as very
narrow rejection filters and Fourier-transform techniques are becoming more
widespread.
Picture of a Raman experiment
Further Information
Science Hypermedia Home
Page
Copyright ©
1996 by Brian M. Tissue
updated 2/24/96

