1996
WSU/NSF Summer Teachers Institute
Department of
Chemical Engineering
Utilization of the CBL
Τ System
Craig T. Gabler
Chemistry/Physics Teacher, Centralia High
School
(cgabler1@esd113.wednet.edu)
in
cooperation with
Dr. Benard Van Wie, Mentor, WSU, and Nancy Davis, Middle
School Teacher, Connell, WA
Table of Contents
I. Introduction
Most pre-college mathematics and science teachers are very familiar with the idea that science represents a body of knowledge, and is a method for expanding that body of knowledge. However, they are likely to be less familiar with the field of engineering and the nature of the work done by engineers. It is this situation that provided the direction for the WSU/NSF Summer Teachers Institute, held on the campus of Washington State University and hosted by the Department of Chemical Engineering. The goal of the Institute was to provide experiences to math and science teachers showing that engineering involves putting matter and energy to use for society, by the practical application of scientific knowledge. Using practical applications, the engineer is typically asked to create a device or system that will do something, better, faster, and/or cheaper. In addition to learning about engineering, the Institute participants were asked to prepare a classroom module that introduces or integrates engineering principles into the existing pre-college curriuculum.
This particular module was created in cooperation with the research being done by Dr. Bernard Van Wie and his team of graduate students. The goal of their research is to develop a multifunctional blood analyzer which utilizes a flow injection analysis approach. The blood analyzer is being designed to perform the eight most common clinical tests rapidly, automatically, less expensively, and in a more compact form than is currently available. To create this blood analyzer the researchers have to first understand the fluid mechanics of the system and determine the reaction kinetics for the system. Because the development of the automated blood analyzer represents the integration of basic scientific knowledge, engineering principles, and applications of technology it is a natural for carry-over to the pre-college classroom.
In the module that follows, the focus is on providing students with a hands-on laboratory investigation of the concentration of glucose or protein in a simulated blood serum. The same reagents as Dr. Van Wies group uses will be utilized, but rather than employing the same elaborate spectrophotometric system, the students will use a Texas Instruments CBL in conjunction with a simple colorimeter. While the protocols for preparing the solutions were created during the Institute, the overall procedure is an adaptation of the colorimeter labs found in the Vernier Software book, Chemistry with CBL. In the investigation the students will prepare a calibration curve, and then from that determine the concentration of their simulated blood serum sample. This application of basic chemistry and mathematics principles is an excellent opportunity for students to learn inference building. As an additional outcome of this investigation students will have the knowledge and skills necessary to conduct an independent research project of their own choosing.
A. Goals
The goal of this module, through the testing of simulated blood serum for glucose and protein, is to provide students with the knowledge and skills associated with an authentic chemical analysis. In addition, students will have the opportunity to acquire the background necessary to carry out an independent research project of their own design.
B. Student Learning Objectives
The student should be able to:
The numbers in parentheses following each objective refer to a Washington State Essential Academic Learning Requirement (EALR). See Appendix D for a list of science EALRs.
C. Prerequisites
In order for the students to conduct these investigations successfully it is necessary that they have previously acquired the knowledge and/or skills to:
II. Teaching Strategies
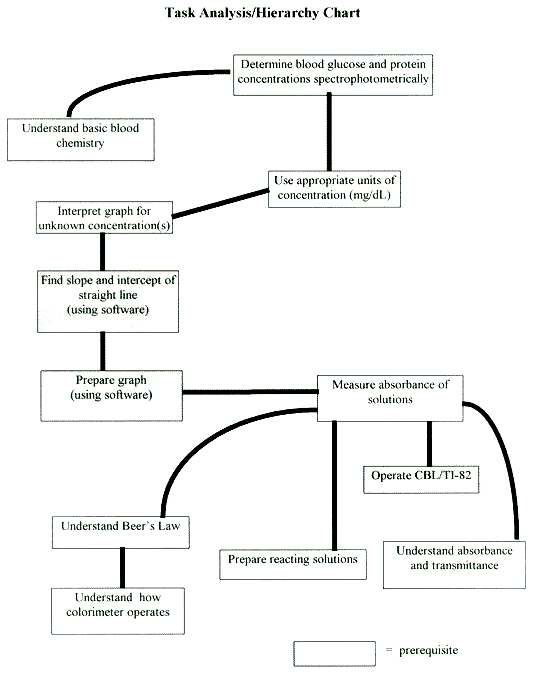
A. Task Analysis
In preparation to teach this module, or any science concept, it is helpful to look at an analysis of the task we are asking the student to perform. In light of the aforementioned student learning objectives, and the stated prerequisites, a task analysis was performed and formatted into a hierarchy chart (see next page). A careful review of this will help you better prepare yourself and the students to carry out this module.
B. Timeline
Day 1: Introduction to Blood Chemistry - glucose & protein levels and symptoms
Day 2: Background on colors, absorbance/transmittance, and Beers Law
Day 3: Pre-lab Day - describe equipment set up, chemical reactions, and safety
Day 4: Lab Day
Day 5: Analysis of Data
Day 6: Post Lab Discussion
Day 7: Lab report due - visit by doctor
C. Teaching Suggestions

- Measurement of the glucose in Pepsi found that a typical Pepsi contained 6.44 g/dL glucose, or 55.8% of the sugar present. This is consistent with food labeling, because the Pepsi contains high fructose sugar, which is generally taken to be 58% glucose and 42% fructose. In the case of skim milk, previous research found it to contain 23 g/L protein.
D. Lab Hints
If you have two CBL/colorimeter setups it is recommended that one setup be used for the protein assay and the other for the glucose assay. A cuvette containing the blank must be provided at each colorimeter. In the case of the glucose assay the blank can be made from 10 drops of water and 2.5 mL of glucose reagent. The protein assay blank should be 8 drops of buffer added to 3.0 mL of Bradford reagent.
The simulated serum samples can be distributed in small test tubes or labeled pipets. The students should be careful to record the identification letter of their serum sample in their lab report. Be sure to notify students that the serum samples are a diluted form.
glucose - 60 mg/dL - Dissolve 0.3 g of glucose in 500 mL of distilled water. Students then dilute this to prepare their glucose solutions. The following example illustrates how the concentrations are computed (this calculation is worth pointing out to students):
glucose reagent - Make up per instructions on bottle, keep refrigerated.
buffer - Dissolve 7.65 g NaCl, 1.26 g Na2HPO4, & 0.1 g NaH2PO4 in 1.0 L.
albumin - 0.1 g/dL - Dissolve 0.05 g Bovine Serum Albumin (BSA) in 0.5 L of buffer. Student concentrations computed the same as for glucose.
Bradford reagent - 0.025 g of Coomassie Blue. Add 125 mL of ethanol. Dissolve well then add 25 mL of concentrated phosphoric acid. Filter. Keep refrigerated. Filter before each use.
Serum Stock Solution - dissolve 0.10 g BSA and 0.15 g glucose (dextrose) in one liter of buffer. Keep refrigerated.
Serums - The following mixtures represent a 1:50 dilution for glucose and a 1:1000 dilution for protein, from what the concentrations would be in straight serum samples. Students must account for this in their calculations for the actual glucose or protein concentration.
Serum Sample
A
B
C
D
mL serum stock solution
90
70
50
30
mL buffer
10
30
50
70
[glucose] mg/dL
13.5
10.5
7.5
4.5
[albumin] mg/dL
9
7
5
3
- Safety
Standard laboratory precautions need to be discussed. All solutions can be disposed of by flushing them down the drain with plenty of water. Emphasize that the serum the students are using is a laboratory prepared serum, not real human or animal serum.
Adaptations
- This investigation could be carried out by each group if sufficient CBL/colorimeter setups are available. This could also allow each group to perform both the glucose assay and the protein assay.
- This investigation can still be carried out if only 1 CBL/colorimeter setup is available by either spreading the lab over two days, or limiting it to one or the other of the assays.
- If the CBL/colorimeter is not available, but a spectrophotometer such as a Spec20 is, the procedure is easily adapted.
Colorimetric Determination
of
Blood Glucose and Protein Levels
When you visit your doctor for a check up, blood may be drawn as part of the examination. Have you ever wondered what they did with that blood, besides counting the number of red blood cells? Depending on what the doctor needs to know, he or she may request that the blood be tested for a number of substances, such as glucose, albumin (a protein), or cholesterol. Have you ever wondered how they measure the concentrations of these different substances? Well, wonder no more.
In this lab you are going to utilize one of the most modern methods for determining blood glucose and blood protein levels. By combining a sample of blood serum with a reagent that turns color in the presence of either the glucose or the protein, and then placing that colored sample in a spectrophotometer, the concentration of the glucose, or protein, can be determined. Your task will be to measure the glucose concentration, or protein concentration, in samples of known concentrations and then use that data to determine the concentration of glucose or protein in a sample of simulated blood serum. As an additional investigation you may choose to measure the glucose concentration of pop, or the protein concentration of milk.
Materials: working with a partner
| cuvettes 13x100 test tubes thin stem pipets 10 mL graduate cylinder 60 mg/dL glucose solution 0.1 g/L albumin solution Bradford reagent Glucose reagent |
TI-82 w/"CHEM" loaded (Vernier software) CBL w/connectors colorimeter |
Procedure:
Part A: Determination of Glucose:
Table 1: Preparation of Glucose Solutions
|
Test tube |
Drops of 60 mg/dL Glucose |
Drops of Water |
Glucose Concentration (mg/dL) |
|
1 |
0 |
20 |
0 |
|
2 |
1 |
19 |
3 |
|
3 |
2 |
18 |
6 |
|
4 |
3 |
17 |
9 |
|
5 |
4 |
16 |
12 |
|
6 |
5 |
15 |
15 |
Part B: Determination of Albumin:
Table 2: Preparation of Albumin Solutions
|
Test tube |
Drops of 0.1 g/dL |
Drops of Buffer |
Albumin |
|
1 |
0 |
20 |
0 |
|
2 |
4 |
16 |
2 |
|
3 |
8 |
12 |
4 |
|
4 |
12 |
8 |
6 |
|
5 |
16 |
4 |
8 |
|
6 |
20 |
0 |
10 |
Analysis:
Conclusion Questions:
Colorimeter Handout
Glucose & Protein
Analysis
Turn on the CBL unit and the TI-82 calculator. Press and select CHEM. Press then press again to go to the CHEM MAIN MENU.
To calibrate the cuvette at 0% and 100% transmittance:
Determination of Blood Glucose and Protein Levels
Name __________________
Period ____
Purpose:
Data:
|
Test tube |
Glucose |
Absorbance of Glucose |
Albumin |
Absorbance of Albumin |
|
1 |
0 |
0 |
||
|
2 |
3 |
2 |
||
|
3 |
6 |
4 |
||
|
4 |
9 |
6 |
||
|
5 |
12 |
8 |
||
|
6 |
15 |
10 |
||
|
serum |
X |
X |
Conclusion Questions:
1.
2.
3.
4.
5.
Determination of Blood Glucose and Protein Levels
Name ___Sample Results_
Period ____
Purpose:
Data:
|
Test tube |
Glucose |
Absorbance of Glucose |
Albumin |
Absorbance of Albumin |
|
1 |
0 |
0 |
0 |
0 |
|
2 |
3 |
.042 |
2 |
.103 |
|
3 |
6 |
.156 |
4 |
.214 |
|
4 |
9 |
.258 |
6 |
.303 |
|
5 |
12 |
.373 |
8 |
.323 |
|
6 |
15 |
.437 |
10 |
.398 |
|
serum sample __A__ |
X |
.081 |
X |
.281 |
Conclusion Questions:
1. See attached graphs. Correlation coefficients indicate good straight line fit, as predicted by Beers Law.
2. glucose: absorbance = 0.0312([glucose]) + 0.0233
albumin: absorbance = 0.0391([albumin]) + 0.0279
3. glucose: .081 = 0.0312([glucose]) + 0.0233, [glucose] = 1.85 mg/dL x 50 = 92.5 mg/dL
albumin: 0.281 = 0.0391([albumin]) + 0.0279, [albumin] = 6.48 mg/dL x1000 = 6.48 g/dL
4. Glucose is in the normal range, while the albumin is a little high. This would seem to indicate that overall the patient is healthy.
5. Answers will depend on the serum samples provided by the teacher.
Appendix A
Blood Chemistry
The analysis of blood is a very important diagnostic tool for the doctor. Typically the doctor will order several basic tests; usually these include assays for blood components like glucose, protein, creatinine, hemoglobin, calcium, sodium, urea, and potassium. Since the focus of this module is on glucose and protein determination this background will be limited to those two components.
Because glucose is a major energy source for the body it is a very important component of the blood. In order to maintain health it is critical that the glucose level in the blood be kept within certain limits. The body has several mechanisms for accomplishing this. The normal range for glucose is 50 mg/dL - 105 mg/dL. Sustained concentrations outside this range are indicative of some sort of abnormal condition or disease.
Hyperglycemia, or elevated levels of glucose are associated with diabetes. It is estimated that as many as 10 million Americans suffer from some form of diabetes. Hyperglycemia is usually due to reduced insulin production, resulting in the bodys inability to metabolize the glucose. While there are two general types of diabetes; the more severe form, Type I - insulin-dependent diabetes mellitus (IDDM), is the one that most people are aware of. This type of diabetes may lead to the patient developing retinopathy with blindness, kidney failure with the need for dialysis, nerve damage, and circulatory problems possibly leading to heart disease and/or stroke.
Hypoglycemia, or low blood sugar, can be associated with such symptoms as weakness, shakiness, sweating, nausea, and rapid pulse. If serum levels drop to < 20 - 30 mg/dL impairment of the central nervous system can occur. In these cases the symptoms may include: confusion, lethargy, and/or loss of consciousness.
In the case of proteins the composition of the blood is much more complex. There have been over 300 proteins identified in plasma, or serum. But the most concentrated of those proteins is albumin, which constitutes 55% - 65% of the total proteins in human serum. This lab focuses on albumin for that reason. Normal albumin levels are in the range of 3.5 - 5.5 g/dL, and total proteins are in the range 6.0 - 8.5 g/dL.
Protein levels are not as strong a diagnostic tools as glucose, but can indicate certain types of dysfunction. Hyperalbuminemia is only of significance in cases of dehydration. Hypoalbumininemia on the other hand can result from liver disease, tissue damage, malnutrition, nephrotic syndrome, diabetes, lupus, or hypertension. If albumin levels are outside the normal range additional tests are required to pinpoint the dysfunction.
Appendix B
Sources for Special Materials
Several special chemicals are needed to carry out the glucose and protein determinations. These chemicals can be obtained from:
Glucose Reagent for glucose determination:
Reagents Applications, Inc.
8225 Mercury CT
San Diego, CA 92111-1203
800-438-6100
Coomassie Brilliant Blue G-250 for Bradford Reagent for protein determination:
Bovine Serum Albumin:
Sigma Chemical Co.
P.O. Box 14508
St. Louis, MO 63178-9916
800-325-3010
Appendix C
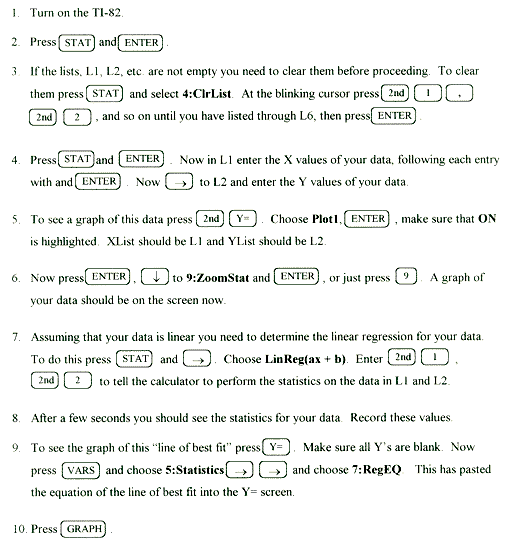
Linear Regression Statistics & Graphing with the TI-82
Appendix D
Washington State
Essential Academic Learning
Requirements
Appendix E
Transparency Masters
On the following pages are absorbance graphs, suitable for making transparencies. These graphs illustrate some of the basic principles of color and light absorbance. Each of the graphs is described below with suggestions on how to use the graph to illustrate the basic principles.
Transparency 1: This graph shows the absorbance of red food color over the wavelength range 400 - 700 nm. The thing to point out here is that the absorbance peak occurs at about 500 nm. This wavelength corresponds to bluish green light. Since the solution absorbs in the blue-green region what we see is red.
Transparency 2: This graph shows the absorbance of yellow food color over the same range of wavelengths. The thing to point out here is that the solution absorbs strongest at about 400 nm, which is the blue region, causing the solution to appear yellow.
Transparency 3: This graph shows the absorbance of green food color over the same range of wavelengths. One thing to notice here is that it absorbs strongly in two regions, the blue region (the same as the yellow) and at about 625 nm in the orange region. Note that the second peak on this graph corresponds nicely to the main peak on the next graph. This suggests that green food color is a mixture of yellow and blue food color.
Transparency 4: This graph shows the absorbance of green food color over the same range of wavelengths. Point out that the strongest absorbance is at about 600 nm, the yellow region of the spectrum, thus causing the solution to appear blue. It may be helpful to place the green, blue, and yellow spectrums together on the overhead so that students can see the simularities.
Transparency 5: This graph shows the absorbance of the dye used in the glucose reagent over the same range of wavelengths. Point out that the solution appears red because it is absorbing in the greenish-blue region (~500 nm), same as red food color. The 470 nm LED in the colorimeter is choosen because it most closely matches the absorbance peak for the dye.
Transparency 6: This graph shows the absorbance of grape pop over the same range of wavelengths. Have students identify the regions in which it absorbs strongly, and then relate that to the color they observe (absorbs ~500 nm - green, and ~625 nm - orange).
Transparency 7: This graph shows the absorbance of orange pop over the same range of wavelengths. Point out that the orange pop absorbs strongly at ~475 nm (greenish-blue) causing the solution to appear orange
Transparency 8: This graph shows the absorbance of the reagent used for the protein assay over the same range of wavelengths. This graphs is actually a composite of two sets of data. Notice that the reagent alone absorbs very strongly at ~450 nm, but when complexed to the protein the absorbance shifts to ~600 nm. It is because of this peak that we choose the 565 nm LED for the protein measurement.
GLUCOSE REACTION
glucose oxidase
b
- D - Glucose + O2 + H2O D - Gluconic Acid + H2O2
peroxidase
H2O2 + Phenol + Reduced Dye Oxidized Dye + H2O