H.M. Heise,
Institut für Spektrochemie und Angewandte
Spektroskopie,
Bunsen-Kirchhoff-Str. 11, D-44139 Dortmund, Germany.
Frequent measurements of blood glucose are essential for diabetic patients without adequate glycaemic control.
Non-invasive monitoring of blood glucose concentration offers many advantages over invasive measurements, since the intermittent tests, which are widely practised by diabetic patients, involve pain and discomfort from frequent finger-pricking.
Many medical instruments, such as magnetic resonance imaging and spectroscopy, rely on the interaction of electromagnetic radiation with body fluids and tissues. The same is valid for infrared (IR) spectroscopy, which finds numerous applications in the medical field [1]. Its great potential lies in the development of small, rugged and moderately priced instrumentation.
The mid- and
near-IR spectral ranges are of special importance for quantitative analysis. In
the mid-IR ![]() significant absorptions can be found, which are very characteristic
and useful for molecular identification, caused by excitation of many different
molecular vibrations assigned to fundamental and combination bands. The near-IR
range covers the wavelengths from 780 to 2500 nm (12800 - 4000 cm-1). In this interval, molecules absorb
radiation for excitation of overtone and combination vibrations. The most
intensive bands are mainly from vibrations of molecular moieties with hydrogen
atoms involved, such as O-H, C-H and N-H. The short-wave near-IR section
(SW-NIR) carries higher overtone bands with even smaller absorptivities, so that
the optical sample pathlength is increased, and aqueous solutions can be
measured, e.g.,with cuvettes of one centimeter pathlength.
significant absorptions can be found, which are very characteristic
and useful for molecular identification, caused by excitation of many different
molecular vibrations assigned to fundamental and combination bands. The near-IR
range covers the wavelengths from 780 to 2500 nm (12800 - 4000 cm-1). In this interval, molecules absorb
radiation for excitation of overtone and combination vibrations. The most
intensive bands are mainly from vibrations of molecular moieties with hydrogen
atoms involved, such as O-H, C-H and N-H. The short-wave near-IR section
(SW-NIR) carries higher overtone bands with even smaller absorptivities, so that
the optical sample pathlength is increased, and aqueous solutions can be
measured, e.g.,with cuvettes of one centimeter pathlength.
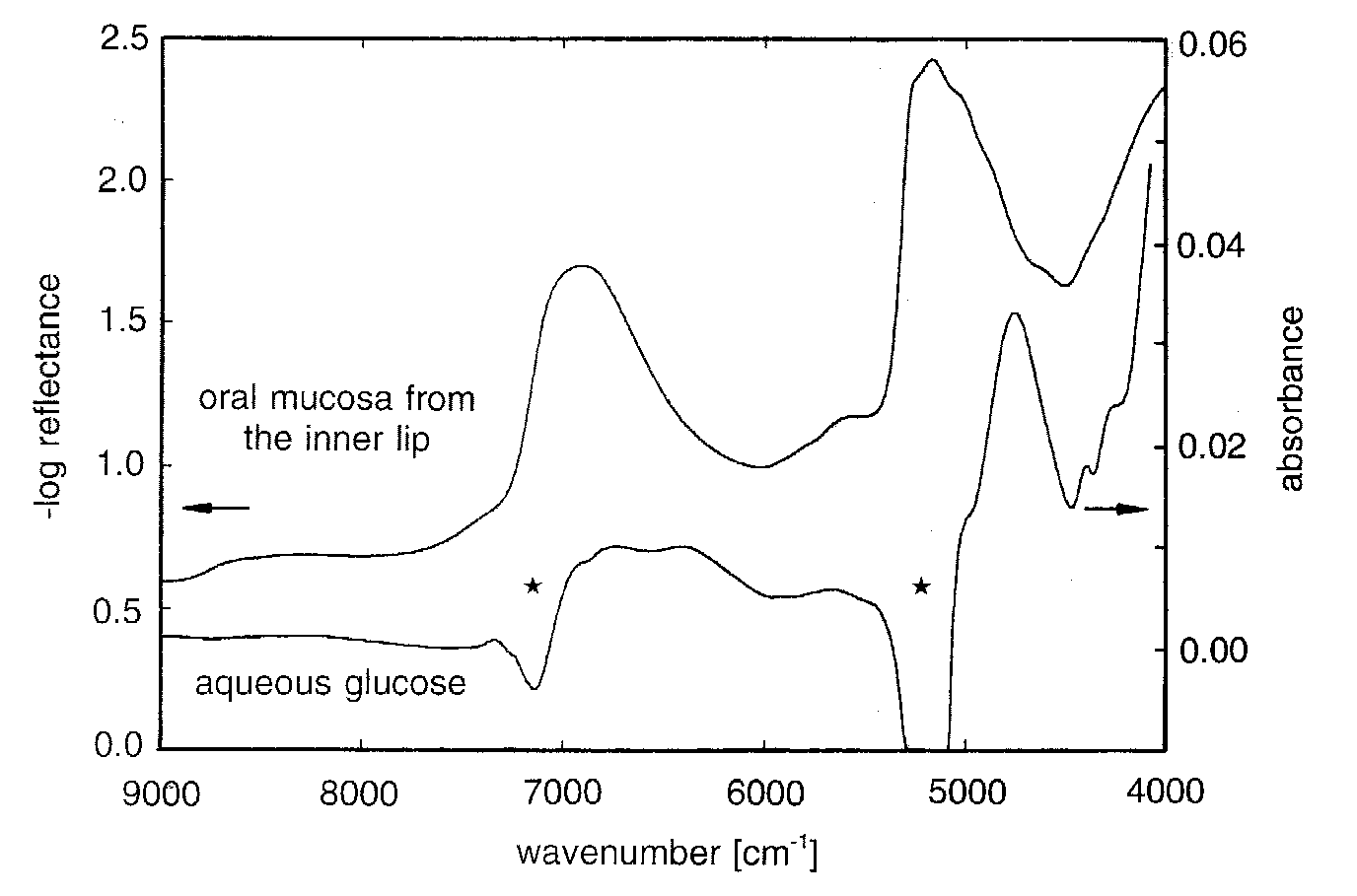 Different
information densities are found for the spectral ranges mentioned with respect
to molecular finger-print signatures. The quantitative investigation of
biofluids, such as whole blood, can be carried out successfully by multivariate
spectrometry, i.e. using broad spectral range information (see Fig. 1), and
novel assays for reagent-free multicomponent analysis have been presented for
clinical chemistry applications.
Different
information densities are found for the spectral ranges mentioned with respect
to molecular finger-print signatures. The quantitative investigation of
biofluids, such as whole blood, can be carried out successfully by multivariate
spectrometry, i.e. using broad spectral range information (see Fig. 1), and
novel assays for reagent-free multicomponent analysis have been presented for
clinical chemistry applications.
Owing to the large water absorptivities in the mid-infrared, penetration of IR-radiation is not sufficient to establish transcutaneous measurements of metabolites in tissue, so a way out of this dilemma is to use near-IR spectroscopy for which the water absorptivities are much smaller. Due to the optical constants of tissue, i.e. the spectral absorption and scattering coefficients, a wavelength dependent penetration depth for such radiation exists. For wavelengths between 600 and 1300 nm (16700 - 7700 cm-1, the so-called therapeutic window) opportunities are available for transmission measurements of body tissues. Another window exists from 1600 to 1850 nm (6250 - 5400 cm-1) between two water absorption bands, which can be used for diffuse reflectance measurements (see Fig. 1). The measurement conditions for the in-vitro analysis of biofluids can be kept better adjusted with regard to temperature stability, sample homogeneity and optical pathlength than the conditions for in-vivo measurements as tissues are of much greater complexity than found for biofluids [2].
Different types of spectrometer are used nowadays for near-IR
spectrometry. Polychromators are available with diode arrays to measure the
whole spectrum simultaneously. Fourier-transform (FT) spectrometers are favored
by us which are based on the Michelson interferometer; their primary information
is coded in the interferogram which has to be Fourier-transformed to obtain the
spectrum. For detection we use photodiodes of different semiconductive
materials, but mainly InSb cooled by liquid nitrogen. A tungsten-halogen lamp is
employed as a thermal source to provide the broad emission of electromagnetic
radiation according to Planck’s law. 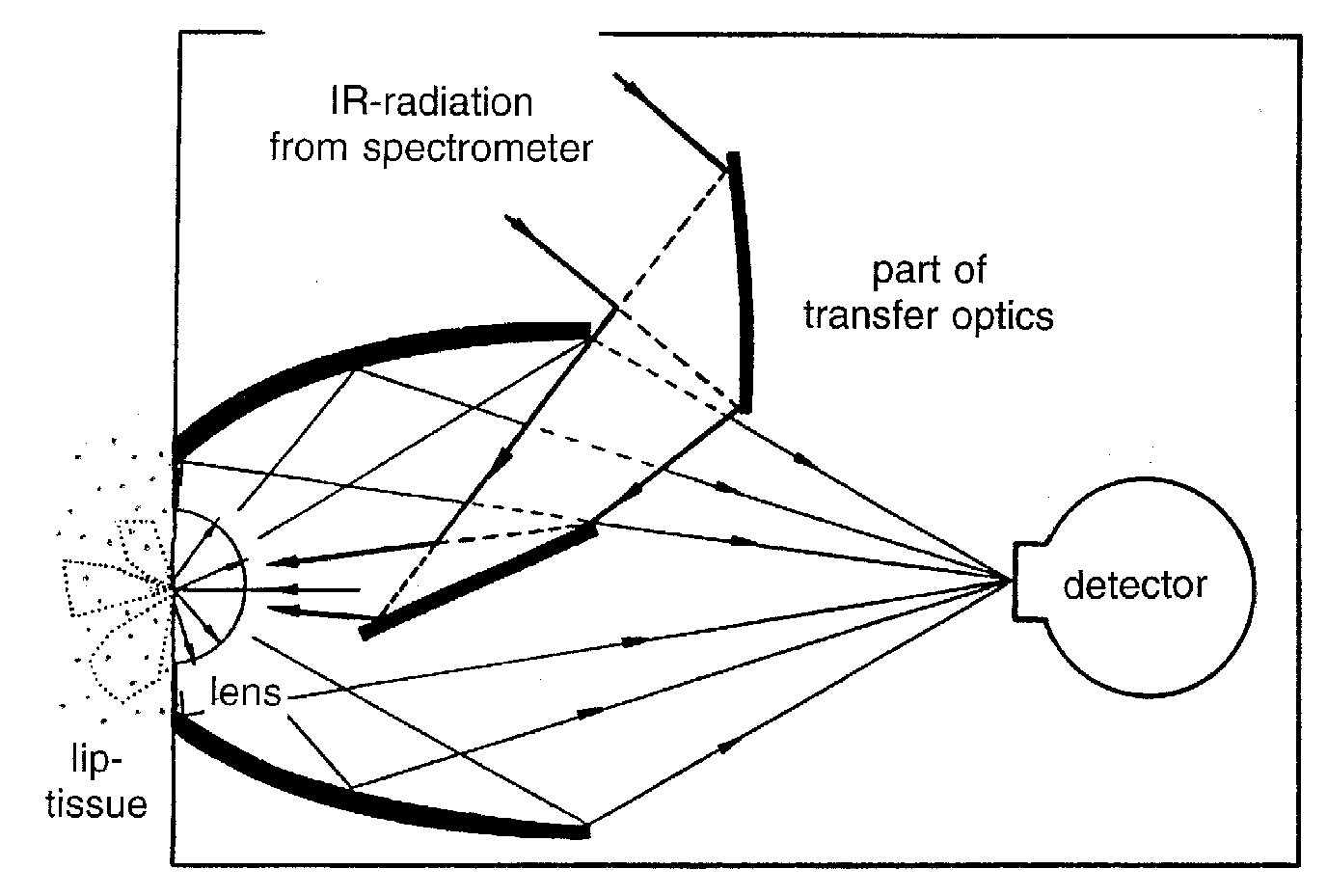
For conventional measurements in transmittance or diffuse reflectance, accessories are based on reflection or fibre optics. We developed a diffuse reflectance accessory for skin tissue measurements optimized to yield the signal/noise-ratio required for glucose sensing(see scheme in Fig. 2). For diffuse reflectance measurements with glucose absorption bands around a wavelength of 1.6 mm, the noise level in the skin spectra should be lower than 10-5 absorbance units to tackle the normal physiological glucose concentration level [1].
For assays of biosamples, it is nearly impossible to have quantitative information on all components contributing to the spectrum, however, regressing concentrations against spectral data, the so-called soft modeling approach works well. For statistical calibrations, it is essential that the calibration data span the range of variations which can influence the spectra of future samples. Often, calibration systems are ill-conditioned due to linear dependencies in the spectral data (so-called collinearity problem). For that reason, special multivariate calibration algorithms such as Partial Least-Squares (PLS) are applied. Recently, a novel strategy for spectral variable selection based on PLS-regression vector choices has been described by us which has been proved to be very successful in our clinical applications, as it leads to improved results compared to full spectrum evaluation previously favoured.
Near-IR diffuse reflectance spectra of the human inner lip were evaluated by us for PLS-calibration using spectral data between 9000 and 5500 cm-1. Single-person experiments were carried out with oral glucose tolerance testing and measurements at random. The average mean squared prediction errors for calibration models based on single-person data were between 2.0 and 2.5 mmol/L (35 - 45 mg/dL), and prediction errors for multi-person calibrations were 10 mg/dL higher. There is currently some controversy about the quality of the statistical calibration models for non-invasive glucose assays. It is well known that the pickup of spurious drift effects can significantly influence the results, especially with regard to continuous monitoring. An appropriate experimental design with randomized sampling is essential for such critical applications when the analytical signals are comparable to the prevailing noise level or signal drift. Recently, clear evidence for the physical effects (i.e. glucose absorptivity) underlying the non-invasive assay using the diffuse reflectance technique was presented [3].
Transcutaneous sensing is information retrieval on chemical changes occuring within the probed body tissue, but the extraction of quantitative analytical information on glucose using in-vivo spectroscopy is complicated by the complexity of the matrix and the physiological variability. Most information on the patho-physiological status of the patient is gained from blood, which constitutes only a relatively small fraction of the tissue volume under spectroscopic investigation. The size of this fraction is not well known and may vary considerably depending on the location and the physiological state of the microvasculature. The intravascular fluid can show a significant spatial variation in glucose concentration, as manifested for example by arterio-venous blood differences. Furthermore, the interstitial and the intracellular fluids also contribute to the aqueous glucose space. The composition of the interstitial fluid is similar to that of blood plasma, but some temporal relationships for the glucose concentration profiles exist.
Additionally for tissue, distinct concentration gradients for glucose can be observed, depending on the capillary density within the microcirculatory bed, blood flow and metabolic rates, although low molecular mass components tend to equilibrate between the different compartments. Further development of non-invasive technology, especially for blood glucose determination, has been presented by us. This is based on time resolved near infrared based on time resolved near infrared spectroscopy allowing the probing of the intravascular arterial fluid space modulated by the heart beat.
Using near-IR plethysmography, similarly to pulse-oximetry, the perturbations from the tissue can be excluded, so that non-invasive blood analysis will be more reliable. In particular, this is important because most medical diagnostic expert systems are based on the analysis of whole blood samples.
However, the development of this technique is still in its infancy [4].
The problem with the non-invasive glucose assay is that the constant, concentration independent prediction error experienced by us is still too large to allow a reliable monitoring in the normal and hypoglycemic concentration range, as spectral data and modeling are still inadequate for practical use. As a guideline, a relative concentration prediction error of 15 % or better over the clinically relevant range between 30 and 350 mg/dL could be considered sufficient, so that further improvements in this promising measurement technique are essential.
References
[1] H.M. Heise, “Medical applications of infrared spectroscopy”, Mikrochim. Acta, Suppl. 14, 67-77 (1997) [2] H.M. Heise, “Near-Infrared Spectrometry for in vivo Glucose Sensing” in Biosensors in the Body - continuous in vivo Monitoring, ed. by D.M. Fraser, John Wiley, Chichester, 79-116 (1997).
[3] H.M. Heise, A. Bittner and R. Marbach, “Clinical Chemistry and Near-Infrared Spectroscopy: Technology for Non-Invasive Glucose Monitoring”, J. Near Infrared Spectrosc., submitted.
[4] H.M. Heise and A. Bittner, “Blood glucose assays based on infrared spectroscopy: alternatives for medical diagnostics”, “Infrared Spectroscopy: New Tool in Medicine”, Proc. SPIE 3257, 2-12 (1998)